Embark on a scientific expedition with “Lewis Structure and Molecular Models Lab Answers,” a comprehensive guide that unlocks the secrets of molecular bonding and unveils the power of visualization in chemistry. This lab empowers students with the tools to decipher the intricate dance of atoms and electrons, laying the foundation for a deeper understanding of the molecular world.
Through hands-on experimentation and expert explanations, this lab provides a captivating immersion into the realm of molecular structures, enabling students to construct accurate Lewis structures and build tangible molecular models. By bridging the gap between theory and practice, this lab fosters a profound appreciation for the interplay of molecular geometry and chemical properties.
1. Introduction
The Lewis structure and molecular models lab is designed to provide students with a hands-on understanding of the fundamental principles of molecular bonding and structure.
The learning objectives of the lab are to:
- Learn the concept of Lewis structures and how to draw them for various molecules.
- Understand the different types of molecular models and how to construct them.
- Analyze the results of the Lewis structure and molecular models lab to gain insights into molecular properties and behavior.
2. Lewis Structures: Lewis Structure And Molecular Models Lab Answers
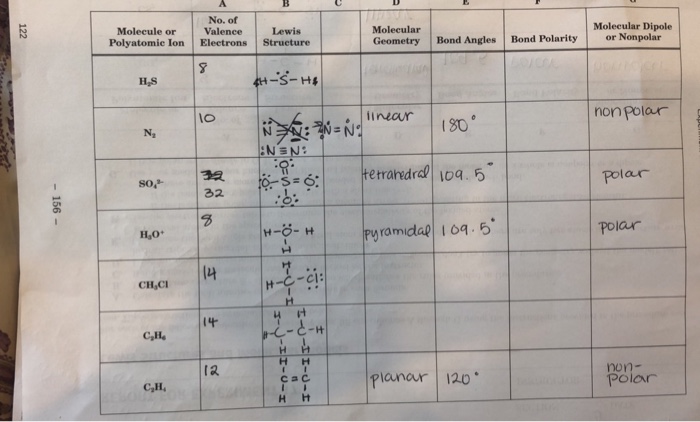
Lewis structures are diagrams that show the arrangement of atoms and electrons in a molecule. They are important because they can be used to predict the bonding and molecular geometry of molecules.
The steps involved in drawing Lewis structures are as follows:
- Determine the total number of valence electrons in the molecule.
- Place the atoms in the molecule and connect them with single bonds.
- Distribute the remaining valence electrons around the atoms as lone pairs.
- Check the octet rule to make sure that each atom has eight valence electrons (or two for hydrogen).
- If the octet rule is not satisfied, adjust the structure by adding double or triple bonds.
3. Molecular Models
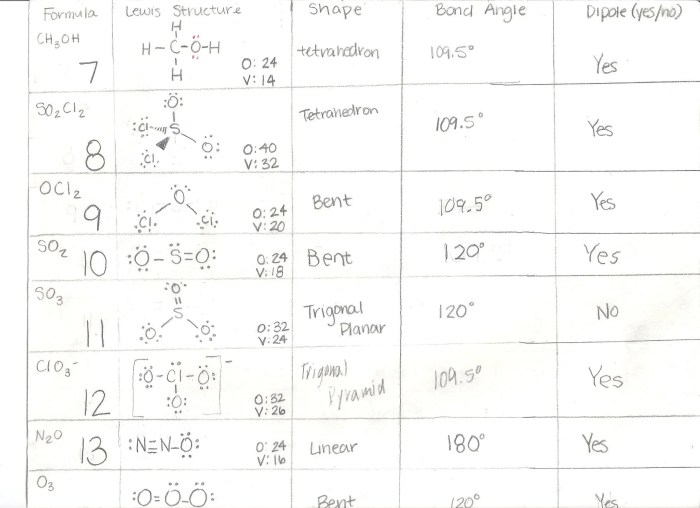
Molecular models are physical representations of molecules. They can be used to visualize the structure of molecules and to understand their properties.
There are different types of molecular models, including:
- Ball-and-stick models: These models use balls to represent atoms and sticks to represent bonds.
- Space-filling models: These models use spheres to represent atoms, which are sized according to the van der Waals radius of the atom.
To construct a ball-and-stick model, follow these steps:
- Gather the appropriate materials, including balls, sticks, and a molecular model kit.
- Identify the atoms in the molecule and their corresponding colors.
- Connect the atoms with sticks according to the molecular structure.
To construct a space-filling model, follow these steps:
- Gather the appropriate materials, including spheres and a molecular model kit.
- Identify the atoms in the molecule and their corresponding colors.
- Attach the spheres to the molecular model kit according to the molecular structure.
4. Data Analysis
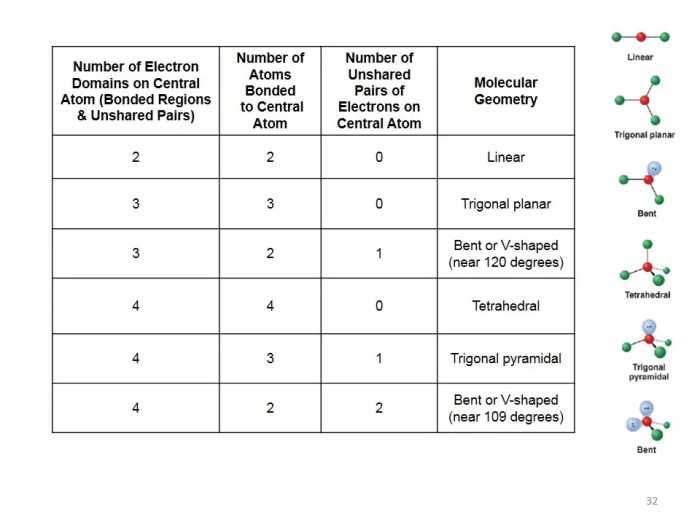
The results of the Lewis structure and molecular models lab can be analyzed to gain insights into molecular properties and behavior.
One method of data analysis is to compare the experimental results with theoretical predictions. For example, the bond lengths and angles in a molecular model can be compared to the values predicted by quantum mechanics.
Another method of data analysis is to use the molecular models to visualize the molecular structure and to understand how it affects the properties of the molecule.
5. Applications
Lewis structures and molecular models are used in a variety of fields, including chemistry, biology, and materials science.
In chemistry, Lewis structures are used to predict the bonding and molecular geometry of molecules. They are also used to understand the reactivity of molecules.
In biology, molecular models are used to visualize the structure of proteins and other biological molecules. They are also used to understand the interactions between molecules in biological systems.
In materials science, molecular models are used to design new materials with specific properties.
FAQ Overview
What is the significance of Lewis structures?
Lewis structures provide a simplified representation of the arrangement of atoms and electrons in a molecule, allowing for a quick visualization of molecular bonding and electron distribution.
How do molecular models contribute to understanding molecular properties?
Molecular models offer a tangible representation of molecules, enabling students to visualize their three-dimensional structures and gain insights into their physical properties, such as polarity, shape, and reactivity.
What are the different types of molecular models?
Common types of molecular models include ball-and-stick models, which represent atoms as spheres connected by sticks, and space-filling models, which depict the relative sizes and shapes of atoms within a molecule.