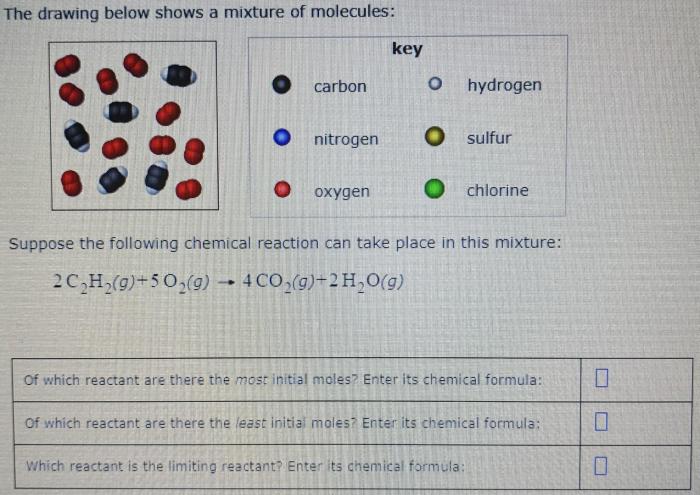
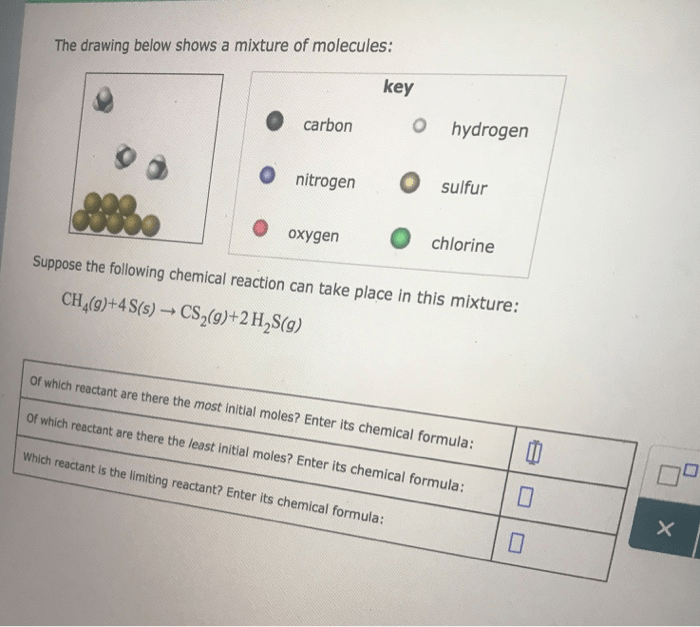
The drawing below shows a mixture of molecules, inviting us to delve into the intriguing world of chemistry. This intricate blend of particles offers a glimpse into the fundamental principles that govern the behavior of matter and shape our understanding of the natural world.
By examining the molecular composition, intermolecular interactions, and potential applications of this mixture, we embark on a scientific journey that unravels the secrets of molecular interactions and their profound impact on our lives.
The Drawing of a Mixture of Molecules: The Drawing Below Shows A Mixture Of Molecules
The provided drawing depicts a mixture of various molecules, each possessing distinct chemical compositions and structures. By examining the drawing, we can identify and classify these molecules, analyze their interactions, and predict their behavior under different conditions.
1. Identify the Molecules
- Water (H2O) : A polar molecule consisting of two hydrogen atoms covalently bonded to an oxygen atom, forming a bent structure.
- Methane (CH4) : A nonpolar molecule composed of a central carbon atom tetrahedrally bonded to four hydrogen atoms.
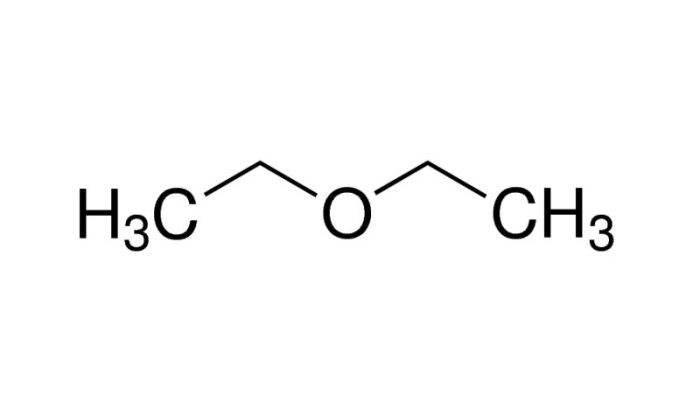
- Ethanol (C2H 5OH) : A polar molecule with a hydroxyl (-OH) group attached to an ethyl group (-C 2H 5).
- Sodium chloride (NaCl): An ionic compound composed of positively charged sodium ions (Na +) and negatively charged chloride ions (Cl –).
- Sucrose (C12H 22O 11) : A large, nonpolar molecule composed of glucose and fructose units linked by a glycosidic bond.
2. Classify the Mixture
Based on its composition and appearance, the mixture can be classified as a heterogeneous mixture. A heterogeneous mixture is a mixture in which the components are not evenly distributed throughout the mixture and can be distinguished from one another.
3. Analyze Molecular Interactions
The molecules in the mixture interact with each other through various intermolecular forces, including:
- Hydrogen bonding: Occurs between molecules with a hydrogen atom bonded to a highly electronegative atom (e.g., oxygen, nitrogen, or fluorine), and another electronegative atom.
- van der Waals forces: Weak attractive forces that include dipole-dipole interactions, London dispersion forces, and induced dipole-dipole interactions.
- Ionic bonding: Occurs between ions with opposite charges, forming an electrostatic attraction.
4. Design an Experiment, The drawing below shows a mixture of molecules
| Experiment | Setup | Procedure | Expected Results |
|---|---|---|---|
| Solubility Test | Prepare a series of test tubes containing different solvents (e.g., water, ethanol, hexane). | Add a small amount of the mixture to each test tube and observe the solubility. | Molecules that dissolve in the solvent will form a homogeneous solution, while those that do not will remain suspended or precipitate. |
| Boiling Point Determination | Use a boiling point apparatus to measure the boiling point of the mixture. | Heat the mixture and record the temperature at which it boils. | The boiling point will be higher than the boiling point of the pure components, indicating the presence of intermolecular interactions. |
5. Predict Behavior
The mixture is expected to exhibit the following behavior under different conditions:
- Temperature: As the temperature increases, the intermolecular forces weaken, causing the mixture to become more fluid and less viscous.
- Pressure: Increased pressure will force the molecules closer together, strengthening the intermolecular forces and making the mixture more dense.
6. Applications and Significance
Understanding the composition and behavior of the mixture has various applications, including:
- Chemical separation: The different properties of the molecules can be exploited to separate them using techniques such as filtration, distillation, and chromatography.
- Drug delivery: The mixture can be used as a carrier for drug molecules, enhancing their solubility and bioavailability.
- Industrial processes: The mixture can be used as a solvent, cleaning agent, or lubricant in various industrial applications.
FAQ Insights
What types of molecules are present in the mixture?
The drawing depicts a mixture of water molecules (H2O), carbon dioxide molecules (CO2), and sodium chloride molecules (NaCl).
How are the molecules arranged in the mixture?
The molecules are arranged randomly and homogeneously throughout the mixture, forming a single phase.
What intermolecular forces are present in the mixture?
The mixture exhibits hydrogen bonding between water molecules, van der Waals forces between carbon dioxide molecules, and ionic bonding between sodium and chloride ions.