Which compound is an isomer of C2H5OC2H5? This question delves into the fascinating realm of isomerism, where molecules with the same molecular formula exhibit distinct structural arrangements and properties. In this exploration, we will unravel the isomers of C2H5OC2H5, examining their structural diversity, physical and chemical characteristics, and their significance in various applications.
C2H5OC2H5, also known as ethyl ether or diethyl ether, is a volatile, flammable liquid with a characteristic sweet odor. It is commonly used as a solvent in laboratories and industries, and as an anesthetic in medical procedures. Understanding the isomers of C2H5OC2H5 is crucial for comprehending their distinct properties and applications.
Isomers of C2H5OC2H5
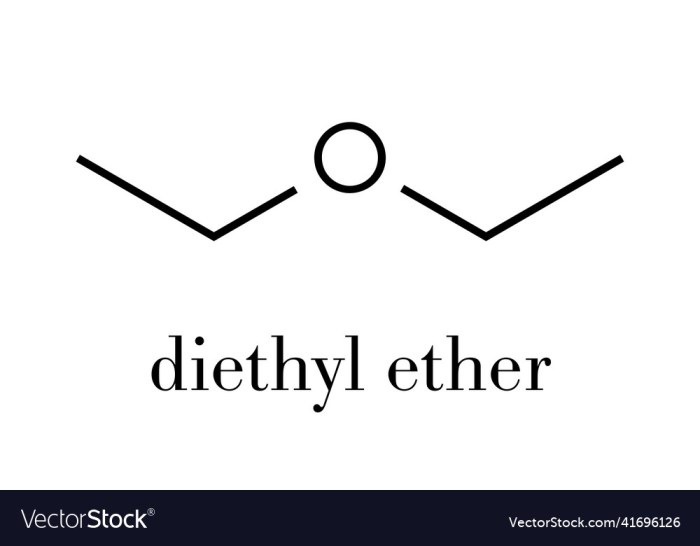
Isomers are compounds that have the same molecular formula but different structural formulas. C2H5OC2H5 has two isomers:
- Ethyl methyl ether(CH3CH2OCH3): The oxygen atom is bonded to the carbon atom in the ethyl group (CH3CH2).
- Dimethyl ether(CH3OCH3): The oxygen atom is bonded to the carbon atom in the methyl group (CH3).
Properties of C2H5OC2H5 and its Isomers
Ethyl methyl ether is a colorless, flammable liquid with a boiling point of 34.6 °C and a melting point of -116.3 °C. Dimethyl ether is also a colorless, flammable gas with a boiling point of -23.6 °C and a melting point of -138.5 °C.
Both ethyl methyl ether and dimethyl ether are soluble in water, but dimethyl ether is more soluble than ethyl methyl ether.
Synthesis of C2H5OC2H5 and its Isomers: Which Compound Is An Isomer Of C2h5oc2h5
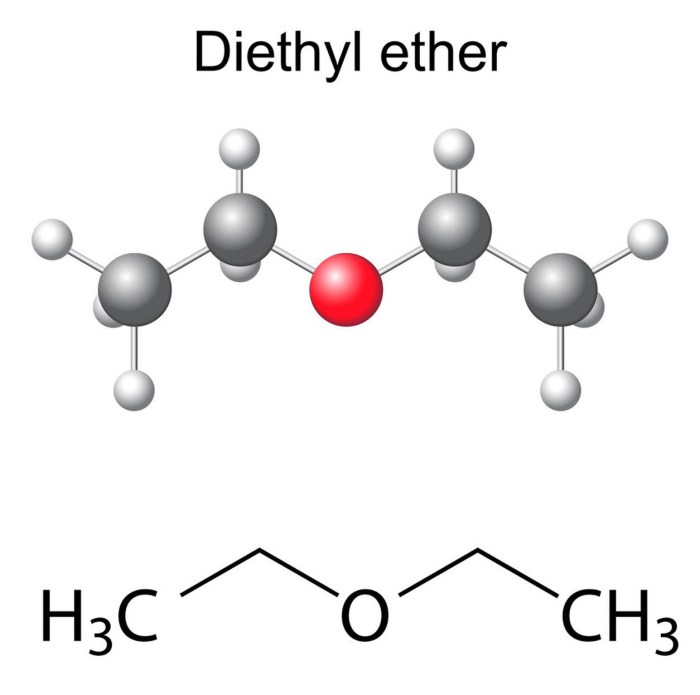
Ethyl methyl ether can be synthesized by the reaction of ethanol with dimethyl sulfate.
Step 1:Ethanol and dimethyl sulfate are mixed in a reaction flask.
Step 2:The reaction mixture is heated to reflux.
Step 3:Ethyl methyl ether is formed as a product of the reaction.
Dimethyl ether can be synthesized by the dehydration of methanol.
Step 1:Methanol is heated in the presence of a catalyst.
Step 2:Dimethyl ether is formed as a product of the reaction.
Applications of C2H5OC2H5 and its Isomers
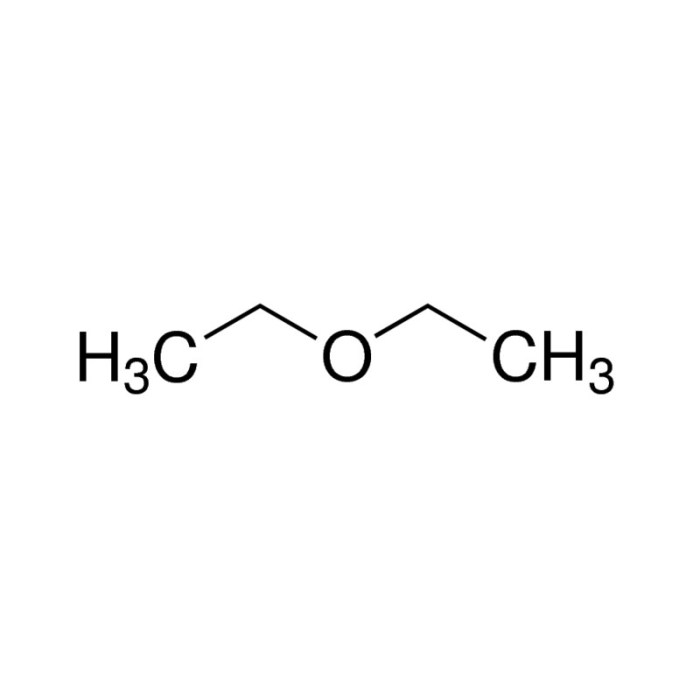
Ethyl methyl ether is used as a solvent in the manufacture of paints, varnishes, and lacquers. It is also used as a propellant in aerosol cans.
Dimethyl ether is used as a fuel in internal combustion engines. It is also used as a propellant in aerosol cans and as a refrigerant.
Safety Considerations for C2H5OC2H5 and its Isomers
Ethyl methyl ether and dimethyl ether are both flammable and can be harmful if inhaled. They should be used in well-ventilated areas and away from open flames.
Ethyl methyl ether is also an irritant and can cause skin and eye irritation. It should be handled with gloves and eye protection.
Top FAQs
What is the molecular formula of ethyl ether?
C2H5OC2H5
How many isomers does C2H5OC2H5 have?
2
What is the structural difference between the isomers of C2H5OC2H5?
The isomers differ in the arrangement of the ethyl groups (-C2H5) around the oxygen atom.